COVID-19 detection kits have proven to be extremely crucial in limiting the spread of the virus and providing immediate medical attention to patients
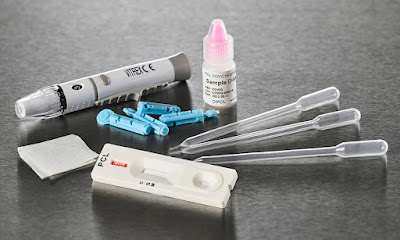
COVID-19 detection kits The COVID-19 Detection Kit is an in vitro multiplex PCR assay that is designed for qualitative SARS-CoV-2 virus nucleic acid detection in upper and lower respiratory specimens. The COVID-19 detection kits are only approved for use in laboratories that have been certified under the Emergency Use Authorization granted by the U.S. Food and Drug Administration (FDA). The kit is not for use in routine clinical practice. The kit is not appropriate for screening or diagnosis of patients. Despite this warning, the COVID-19 detection kits remain the gold standard for determining the presence of the disease. This is a critical first step in managing a COVID-19 outbreak. According to the World Health Organization (WHO), as of November 2021, over 258 million cases of COVID-19 have been confirmed with over 5 million people dying due to the same. While testing remains a crucial st...

